Diane’s farewell message
After 52 years at WAMU, Diane Rehm says goodbye.
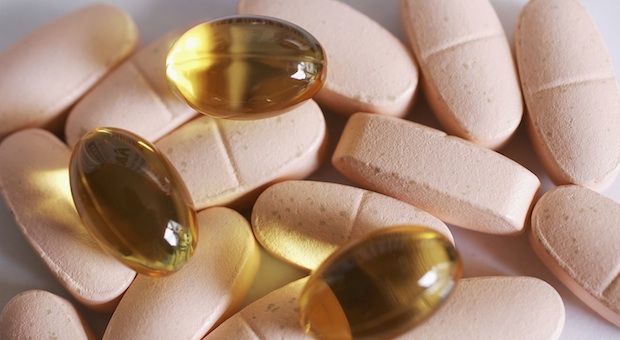
It’s been 20 years since lawmakers first butted heads over the regulation of dietary supplements. The question was: should vitamins, minerals and other naturally derived nutritional products be regulated like a food or like a pharmaceutical? The answer was: like a food. That set the stage for what critics call a “wild west” environment in which companies make health claims and bring products to market without proof of safety or effectiveness. Yet, those concerns have not slowed growth. Sixty-eight percent of American adults use dietary supplements, while annual sales have ballooned to $32 billion a year. Still, the debate over safety and regulation continues. Diane and her guests discuss concerns about the safety and regulation of the supplement industry.
MS. DIANE REHMThanks for joining us. I'm Diane Rehm. A long simmering debate about safety and regulation of dietary supplements bubbled up recently during a high profile Senate hearing featuring TV's Dr. Oz. 68 percent of Americans say they take a supplement. It's a category that includes vitamins, minerals, protein powders and probiotics. But critics warn unknown hazards lurk within pills, powders and capsules that we turn to for maintaining health and well-being.
MS. DIANE REHMJoining me to talk about supplements, Daniel Fabricant of the Natural Products Association. And Julie Rovner of Kaiser Health News. Joining us from a studio at Harvard, Dr. Pieter Cohen of the Cambridge Health Alliance and the Harvard Medical School. I know many of you will want to chime in. Give us a call. 800-433-8850. Send us an email to drshow@wamu.org. Follow us on Facebook or Twitter. And welcome to all of you.
MS. JULIE ROVNERThanks for having us.
MR. DANIEL FABRICANTThanks, Diane.
DR. PIETER COHENThank you.
REHMGood to see you all. Julie, I'm going to start with the most complicated question of all for you. How are these dietary supplements actually regulated?
ROVNERWell, what's important to remember is that they are regulated in a different way, really, from either food or drugs. This was created in a 1994 law that was actually a response to a 1990 law, the Nutrition Labeling Law, that I think people remember. That's what's responsible for all those labels that you see on the sides of food packages. But there was a section in there about dietary supplements. David Kessler, rather famously, activist, FDA Commissioner, took that rather seriously and was going to go ahead and regulate dietary supplements, mostly as drugs.
ROVNERAnd there was a huge outcry.
REHMHuge.
ROVNEREnormous lobbying effort.
REHMI still remember it.
ROVNERI remember it, too. I covered it.
REHMYeah.
ROVNERAnd so, in 1994, Congress passed the Dietary Supplement Health and Education Act. And basically, what it says is that the FDA has very limited regulatory powers over diet supplements. They are not like drugs. They do not have to prove that they are safe and effective before they go to market.
REHMSo no testing?
ROVNERWell, no. There should be testing, and there are outside groups, actually, that do the testing, but the makers themselves do -- if it is a new product, a product that hasn't previously been in food, they have to tell the FDA 75 days in advance. And they do have to show their own evidence of safety, but the FDA can't stop it and the FDA can only go in after the fact if they find that a supplement is dangerous. And we've had some enormous fights over this, including things like Ephedra back in the early 2000s.
ROVNERWhich was banned and then unbanned and then banned again. So, and then, of course, there's also the Federal Trade Commission, and that's what the hearings were about a couple of weeks ago. They basically police, you know, what are considered fraudulent claims by makers of dietary supplements.
REHMAnd how did Dr. Oz figure into all of this?
ROVNERWell, Dr. Oz, on his show, had talked about, I think it was green coffee extract, if I'm not mistaken. And according to Dr. Oz's testimony, he said that some of his comments were taken out of context by people who were advertising them. That he doesn't himself endorse the products that are being advertised, using his name. But there is an enormous amount of advertising on the internet, on TV. I mean, you can see it everywhere, you know, using Dr. Oz's name. Dr. Oz was rather contrite, said he was sorry, but the Senate committee, let's say, was not amused.
REHMAll right. And to you, Dr. Cohen at Harvard. What concerns do you have about the current regulatory scene as far as these supplements are concerned?
COHENDiane, the concerns are actually many. Unfortunately, the current framework that Julie so nicely summarized has not panned out as we'd hoped. So, back in 1994, it was thought that this would be the best way to provide consumers with plentiful vitamins and safe botanicals without FDA meddling or regulatory meddling. But, in fact, because of a combination of factors, the industry has just ballooned. So, we've moved from a few thousand supplements in 1994 to, Dan will know for certain, but last count I heard, 85,000 today.
COHENAnd what's being introduced is often done without any safety, safety data analysis, whatsoever. To make matters worse, the framework states that the -- all supplements, as long as you label something as a dietary supplement, that, and you put it in a bottle, put it on a store shelf, the FDA's job is to find those dangerous ones and remove them from the market. But the problem is that the FDA has no functioning system to actually remove dangerous -- find dangerous supplements. And remove them swiftly from the market.
COHENAnd we've seen tremendous delays that have led to hospitalizations and deaths, because of this lack of monitoring.
REHMAre you saying that the FDA simply does not have the wherewithal to find those dangerous supplements? Or that these supplements are getting on to the market only on the word of the manufacturer?
COHENDiane, you're correct on both points. So, they're getting on the store shelves, just on the manufacturer's word, but then, the FDA doesn't have the mechanisms to remove the dangerous supplements. And then, other major problems with the law are terrible lack of transparency. When consumers go to stores, they would be able to make wise decisions for themselves if they knew what was in the bottle. But the law doesn't require basic information like the amount of an ingredient listed on the bottle.
COHENOr the adverse effects that are known to be caused by the ingredient to be listed on the bottle.
REHMAll right. Let me turn now to Daniel Fabricant, because you were head regulator of dietary supplements for the FDA. Are Dr. Cohen's concerns valid ones?
FABRICANTI think if you're talking about if the laws that are in place are sufficient, I think the answer is yes. We use those tools repeatedly at the agency. You know, you have to disclose on dietary supplements all added ingredients. For example, with conventional foods, you don't. Things like caffeine don't have to be disclosed on conventional foods. They do on dietary supplements. If you're talking about the agency's will power and resources, well, I think that's a problem across the board in all government right now.
FABRICANTAnd I think that that's, you know, a challenging issue. You know, we brought a lot of cases that were had for the first time, when I was at the agency. And I think that that's -- when you do that, you may be uncovering somewhat of a learned helplessness at the agency, and you want to push that aside. But I think the tools were there. Clearly, we use them. We handed out lifetimes of jail sentences and took billions of dollars of products off the shelf. So, I think that...
REHMBut that was back then. What's happening now?
FABRICANTWell, it was only a few months ago, so there are still actions being processed. You know, how the agency's gonna proceed is, for a lot of very good legal reasons, no longer my business. But I think, you know, certainly, it needs to be a priority. I think one concern is you've got tainted products masquerading as dietary supplements. That's a direct public health concern. Those aren't dietary supplements. They're not part of the industry. You've had over 400 recalls of those since 2008, of products generally containing the diet drug Sevutarine (sp?), which was removed from the market for cardiovascular complications.
FABRICANTViagra analogues. And so, with 400 recalls, but only maybe 10 to 15 prosecutions, what kind of message are the agencies sending, is...
REHMWell, and the question is, with thousands and thousands and thousands of these supplements, 400 prosecutions sounds...
FABRICANTIt wasn't 400.
REHMOr 400 removals sounds very slight.
FABRICANTWell, and again, these are for products that were tainted. These aren't even dietary supplements. You can't put a drug in anything without it being approved the agency. So, the fact of the matter -- these were out there, and there have only been 10 to 15 prosecutions. That behavior needs to be deterred, no matter what the commodity is.
REHMJulie, what about dietary supplements coming in, say, from China? Vitamin C, other -- Echinacea, made in China is on the label.
ROVNEROne of the difficulties, and it's not just the ones made in China, but one of the difficulties in general with diet supplements is that there are many outside groups that test them and find that many of them don't have what their labels say they have. So, regardless of whether or not they work, do whatever.
REHMGive me an example.
ROVNERThe claims are, well, the group Consumerlab.com does some of these testings, and I think they just did joint supplements. And they found, you know, a number of them passed and contained what they said they did, and a number of them just did not. They did not contain the amount of the active ingredient that they said they did, whether it was glucosamine or chondroitin or whatever that are typical in joint supplements. This is a continuing issue about the -- you know, whether, it's not even whether those supplements do what they purport to. It's whether the bottle you buy actually contains the supplement that you think.
REHMSo, you must be more than a little concerned about that, Dan.
FABRICANTWell, I actually used to work for Consumer Lab, and one of the reasons I stopped working there is there science wasn't peer reviewed. Firms are required by law to meet the good manufacturing practices, similar to what's in pharmaceutical good manufacturing practices, where they have to test to ensure the identity, strength, potency, purity of their ingredients. If they don't, it already is illegal. And the agency has taken action. We took quite a bit when I was there for firms that didn't meet GMPs. Didn't test.
FABRICANTSo, you know, there are those problems. I think it's a resource issue. Is the agency inspecting enough firms to get at that problem, if it is a problem at all? And more to the point is where is the science on some of these, like with Consumer Lab and other groups?
MS. SUSAN DENTZERDaniel Fabricant. He is CEO of the Natural Products Association. He's former Director of the Division of Dietary Supplements at the Food and Drug Administration. Short break here. We'll be right back.
REHMAnd welcome back. We're talking in this hour about dietary supplements, what's in them, how they are regulated, who makes them, how we as consumers can know what's really in them. Julie Rovner is here. She was talking about some tests which indicated that active ingredients that were supposed to be in a health supplement were simply not there. Also here, Daniel Fabricant. He's CEO of the Natural Products Association. And joining us from Harvard, Dr. Pieter Cohen. He's a general internist at the Cambridge Health Alliance.
REHMDr. Cohen, I know before the break you wanted to add to something Dan was saying.
COHENI do because Dan is absolutely correct that -- and he knows much better than I do that there are good manufacturing practices that are required by law. So that's the example of a basic fundamental part of the law that exists. But what Dan also knows is that when he was running the supplement team, they found a tremendous number of companies that were not compliant with these basic rules.
COHENSo in other words, this would be like trying to bake banana bread in your kitchen. But instead of taking a banana, mashing it up and putting it in the banana bread, you're buying mush from China. And without testing if it actually is banana, pouring it in.
REHMThat doesn't sound too good, Dr. Cohen. Are you suggesting that that is what some manufacturers are actually doing?
COHENNot only am I suggesting. This is a well-known fact. Julie hit on a great example. There's been with chondroitin recently the supplement companies in America might've thought they were buying the real product at a bargain price, were pouring in the white powder. But it turned out it was a food additive filler instead. So that's a great example.
COHENAnd I agree with Dan too that relying on research that isn't peer reviewed can be sketchy. So let's turn to some peer reviewed research to find out what about the final product. And what we find is that on products as straightforward as vitamin D to those as complicated as herbal extracts, what's listed on the label is not the amount that's found in the bottle.
REHMSo Dan Fabricant, what is the point and the responsibility of the natural products association in regard to what just occurred from Dr. Cohen?
FABRICANTWell, I think first and foremost we educate our members on what to do to be compliant with all the laws, including GMPs, which requires that they test and ensure their products meet their specification, meet what's on the label. So that's a big part of what we do. We also...
REHMWhy do they have to be educated? Why aren't they simply putting in there what is supposed to be in there?
FABRICANTWell, they have to be educated on the scientific aspects of testing. As Pieter pointed out, it is -- there's a component of this. It's not trivial. Testing for ingredients in botanicals is a very -- it's a highly skilled area. So there are some testing statistics, things you see -- you see education in all FDA-regulated industries, whether it's manufacture of pharmaceuticals, over the counter drugs, medical devices. I think it's fairly standard and it's what any responsible association does.
REHMSo that is your primary function?
FABRICANTWell, it's a big part of our function but our other function is to -- you know, again, we want to see folks who aren't -- and again, when I was at the agency, we didn't discriminate based on size. A lot of the folks we took action against aren't members of associations. I think one of the key things -- and Pieter and I have had this discussion before -- is, you know, you follow the claims on products.
FABRICANTIf claims sound too good to be true, do we think those same folks -- irresponsible folks are going to invest in quality? No. So I think we want to see the book thrown at those folks. We want to see the agency use its full force, which it has under the law, to get those folks out of business. Now, at the same time with that said, are there areas in GMPs even on the drug side that are scientifically debatable? Sure. So we as an association need to always be mindful of what those issues are.
REHMJulie.
ROVNERI think one thing that's important to mention is that even though this law is almost 20 years old, these final regulations on the good manufacturing practice is what basically requires the companies to make sure that what's on the label is what's in the bottle didn't come out until 2007. And even then they were phased in over several years for the smallest companies. So they've actually only been enforced really for the last couple of years.
REHMLet me read to you an email from Rob who says, "Ten years ago I used to work out body building and I would take the whey powder and Creatine supplement for working out. Five years ago I lost my left kidney and it was due to the Creatine. Is there any confirmed link to using Creatine or Creatine and kidney damage," Dr. Cohen?
COHENWell, I'll comment briefly that, yes, there have been rare -- very rare case reports of that happening. So it's not out of the realm of possibility. But I think we should step back a little bit because Rob's very question raises the issue. If we don't know -- if people didn't test what was in his Creatine, we don't know what might've caused the damage to his body, to his kidneys in this case.
COHENSo I'd like to first make sure that whenever consumers go to the store to purchase protein powder, amino acids, pre-workout supplements, you name it, that what's on the label is what's in the pills and powder.
REHMBut how can we as consumers really come to judge that? We might go to a Whole Foods or a store like that which says they sell all natural ingredients. But how do we as consumers know, Dr. Cohen?
COHENDiane, your point is so well taken. This is exactly why we need good regulation in the realm of dietary supplements because we're talking -- we're not talking about food. We're not talking about tomatoes that are sitting there spoiling on the store shelves, they taste bitter. We're talking about powder we do not see that we swallow without tasting. So therefore there's no other way but this is the perfect opportunity to have regulations. And I don't even think we need a ton more regulations. We need good regulations. And the current regulations are not working.
REHMWhy are they not working?
COHENWell, another major problem that we haven't talked about yet is something called structure function claims. This means that the -- when the law was passed in 1994, the congress gave the green light to companies to promote their pills and powder for just about anything without a single human study being performed. So that's why Dr. Oz and at GNC and you name it have tons of products that say, this'll burn your fat. This'll help you lose weight and melt away your pounds. All that is completely endorsed by the current regulatory framework.
COHENSo we have a situation where we can sell products as if they have these wonderful functions like improve your workouts. But in reality there's not a whit of data to support that means that we're going to be putting the consumers at risk and in the dark. But I do...
REHMOkay. So excuse me. I think what you're saying -- I want to translate for myself. I think what you're saying is that while the ingredients themselves need to be regulated, the claims that these drugs make, these supplements make, they can be exaggerated. Julie.
ROVNERYes. Well, they all have to -- and I'm sure everybody has seen this on the labels of all of these products that say, this statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease. That's because if they actually said that it would be a drug and it would have to be regulated as a drug. So supplements all carry that disclaimer.
REHMBut what can they say?
ROVNERThey can, however, as we were just hearing, make claims about structure and function. That would be as benign as calcium builds strong bones. That's your sort of classic example if it's a calcium supplement. This is what calcium does. What they're not going to say is that this calcium supplement's going to cure your osteoporosis. That's supposed to be the line. As we've seen, the line gets crossed pretty often. That's a...
REHMThe line gets blurred.
ROVNERIt -- yes.
REHMAnd what can you say about that, Dan?
FABRICANTYou can actually make a health claim, it's been approved by the government, about calcium, vitamin D and prevention of osteoporosis. So I think the point is people want information about products. And Dr. Cohen is, I think, incorrect in two areas. One, I think the people that are sitting in jail because of FDA regulations will have a different take, that there aren't regulations too. You have to have substantiation for the claims you make.
FABRICANTFTC -- and this is the whole basis of that Dr. Oz hearing is, you know, fraud is fraud. No one wants fraud. And FTC's largely responsible when that hits the airwaves in advertising or TV or print. So people are making claims they can't support. FTC has levied heavy fines against them, disgorge them of their assets and people have even gotten jail time. So the law requires that you have substantiation.
FABRICANTAnd I think, you know, looking at regulation only as premarket approval -- you know, aspirin's never been premarket approved. So that system of there's only one way to regulate and it's got to be premarket approval because it's omnipresent is completely -- it's not reality when you look at the totality of food and drug law.
FABRICANTYou know, I don't think that the costs and things like that -- for example calcium, do we need 14 years worth of studies and $1.2 billion worth of clinical trials to tell us that calcium builds strong bones? No. And we're not saying that calcium is used as a drug. The intended use is not as a drug so why would the studies at the regulatory framework be the same way?
REHMWell, because I want to know if I take that pill that makes that statement calcium builds strong bones, am I going to improve the strength of my bones? It's my understanding that calcium growth does not continue after about the age of 18. So why am I taking those calcium supplements?
ROVNERAnd of course now there are new recommendations about calcium, which is, you know, the science changes all the time. So that's -- which is another issue with some of these.
REHMDr. Cohen, can you describe some recent cases of reported harm? I gather there was one with Ayurvedic Remedies that got some attention back in 2003.
COHENYes. So there's been problems with heavy metal poisoning due to some remedies but that's not that common these days. The sort of things we're seeing -- and this story will tell us a little bit about how these companies are punished and regulated. Let's take a company called USP Labs which was heavily marketing a new stimulant called DMAA. After this new stimulant, which shouldn't have been in supplements in the first place, was found to cause heart disease, linked to several deaths, bleeding strokes, the FDA moved very aggressively to remove it from the market.
COHENWell, what the company did, USP labs did, they reformulated their weight loss supplement with a brand new ingredient that they decided to introduce called (word?) . That new ingredient was the one that led to an outbreak in Hawaii of liver inflammation, hepatitis which led to 47 hospitalizations, three liver transplants and one death. So the idea that this company that just was doing something inappropriate would then move immediately to another dangerous supplement is an example of what goes on in the supplement industry.
REHMAnd you're listening to "The Diane Rehm Show." Dan Fabricant, how would your organization respond to issues like that?
FABRICANTWell, it's one of the reasons why our organization, in response industry, supported the introduction of an adverse event reporting requirement for dietary supplements back in 2006. And actually that incident Dr. Cohen spoke about was my case at FDA. And the way we were able to act was by the legal requirement to have adverse event reports. If we didn't have those adverse event reports at FDA people may still be injured by the product.
FABRICANTI liken it to -- and dietary supplements are the only category of food that has that requirement for adverse event reports. We saw recently people were sick -- 516 people got sick because of chicken with salmonella. It was a multidrug-resistant salmonella bug. And so because there was no adverse event reporting system for that, it lingered and lingered and lingered until the government finally acted. So, again, I think that's why, you know, we supported the amending of the Food Drug Cosmetic Act. And it's the right thing to do and it gave the agency the tool to take action.
REHMAll right. And joining us now, let's see where she is, if I can get all these papers together. I think we'll have to wait for just a minute until after the break. Julie, tell us about Purity First Health Products in Farmingdale, N.Y. Apparently they had vitamins and minerals found to contain two powerful anabolic steroids. And some women who took them developed masculinizing symptoms.
ROVNERWell, I'm actually not familiar with that particular case but I do know that one of the big problems with diet supplements is what to do about storage. There's actually a bill working its way through congress right now, bipartisan bill. Apparently what people have been doing is kind of reverse engineering steroids to try to make them sort of not drugs and then putting them back together and selling them as supplements. And it's basically a loophole in the bill that's moving through congress now. It closed that loophole.
ROVNERThis has been an issue but, you know, as you mentioned, you know, one of the issues with -- and we should say, this is not -- all supplements are not -- most supplements it's -- you know, it's a very small percentage but the problem is it's hard to know which ones.
REHMWell, and given the size and breadth and numbers of these supplements that people take, I mean, even a small percentage adds up to an awful lot of problems, Dr. Cohen.
COHENThe problem in terms of having anabolic steroids, designer steroids in supplements is a small but from a health perspective very important subset. But the issues that we've been talking about today involve the great majority of supplements. Not complying with manufacturing practices, not having the quantity, not disclosing adverse events.
COHENLet's take a look at something as common as calcium. On no calcium tablet that I've seen does it say, taking this calcium supplement will increase your risk of kidney stones. But if you have your calcium in food, you won't have that problem. So these are the kind of information I think consumers want to know, but you can't get them currently from supplement labels.
REHMAnd that is the voice of Dr. Pieter Cohen, a general internist at Harvard University Medical School. Short break here. Your calls when we come back. Stay with us.
REHMAnd welcome back. Here's a fascinating question from Pamela. Are makers of supplements required to warn whether a given supplement has dangerous interactions with, for example, prescription medicine? Do they warn when a supplement is dangerous with certain conditions a consumer may already have? Julie.
ROVNERWell, they're not required to. And this is an issue. And I think more and more primary care doctors do ask, you know, when they ask you, you go down the list with your doctor.
REHMRight.
ROVNERWhat drugs you take. They also ask what supplements you take, because there are some known interactions.
REHMBut there could be good supplements and not so good supplements, couldn't it?
ROVNERYeah, but I think in this case, we're talking about supplement that actually are...
REHMDo what they're supposed to do.
ROVNER...what purport to be in the bottle. And there has been -- the one thing that we've seen in the 20 years since the law passed is an enormous amount of research. Part of this is at the NIH Office of Dietary Supplements. And they want to see more peer reviewed articles on which of these supplements work and which of them don't. And how they interact with other things, with people with other chronic conditions and other prescription drugs. So, there is, at least, a much bigger body of evidence than there was 20 years ago.
REHMDr. Cohen.
COHENI completely agree with Julie. And that see in clinic all the time the challenge is that if my patient's taking St. John's Wort, which might interact with many different prescription medications, as they move from one supplier to a different one, or from one month to another, the product is likely changing so much that that adds another complexity to it. So, not only is the supplement not giving that information to the consumer, but as a physician, we're at a loss to even try to figure out what's going on because of the poor quality of the actual supplements.
REHMAll right. And joining us now, by phone, from Philadelphia, is Sarah Erush. She's the Clinical Manager in the Pharmacy Department at Children's Hospital of Philadelphia. Hi Sarah. Thanks for joining us.
MS. SARAH ERUSHHi. Thanks for having me.
REHMCertainly. Tell us about your hospital's policy regarding dietary supplements.
ERUSHWell, I think because of all the things that have been discussed here, we had a growing concern about some of the products that our patients were being admitted on and taking when they came in to the hospital. As well as some of the vitamin products and amino acid supplements, et cetera, that we were stocking for our patients. It became clear to us that there wasn't the kind of quality regulation that we were used to in handling pharmaceuticals. So that we had to put something else in place, to assure that our patients would get quality products and would not be harmed by them.
ERUSHSo, it was basically a two pronged approach that we took. The first being for patients who came into the hospital taking dietary supplements. Our tenet here is to try to have a discussion with them about our concerns regarding these products and discourage them from using them. Especially during the hospitalization. And oftentimes, patients and families, when we sit down and talk to them about all these concerns that you've all nicely discussed already, they're quite willing to put them aside during the hospitalization.
REHMSo, tell me what it was that led to your making this change.
ERUSHWe've had a couple examples of children admitted to our hospital that we were able to determine that the problem they were experiencing, at that time, was due to a dietary supplement they were taking. So, for example, we had one admitted with pancreatitis, or an inflammation of their pancreas, and they were taking 40 dietary supplements, about 20 of which we couldn't even identify the contents of. And we were able to finally convince the family to discourage the use of these products, and once the products were discontinued, the patient's pancreatitis resolved. We were fairly certain it was due to something they were taking, but we couldn't pinpoint exactly which one.
REHMYou know, I'm fascinated to hear that a parent was allowing a child to take 40 different supplements. What do you -- I realize you may not be a psychiatrist, but what do you think is going on in the parent's mind that leads that parent to administer that number of supplements.
ERUSHI think we see a very potentially chronically ill population. Children with diseases that are difficult to treat and to maintain good health with. And in their frustration with the fact that their child may not be responding to the medications as we would hope they would, they feel that dietary supplements are potentially helpful and not going to hurt. You know, a lot of the times, thought is, it might help and it's not gonna hurt. And what we try to educate the families about is that that's not true.
ERUSHWe don't have a lot of data that it will help. Sometimes we have zero data that it will help, but we certainly do have plenty of data that they might be harmful.
REHMSo, not even vitamins are on your approved list?
ERUSHWe have about 30 products that we would consider dietary supplements, that are considered dietary supplements that we have on our hospital formulary, or the list of approved products. And to get on that list, they need to go through the same process that our prescription medications do, which mean we evaluate them very carefully for efficacy and safety. The problem is, with dietary supplements, is there's oftentimes not that data out there about efficacy and safety. And then on top of that, another problem exists is we have no guarantee of quality.
ERUSHWhen we have a prescription product, we have a very clear guarantee that if it's FDA approved, it's a quality product, and we don't have to be concerned about that. For the dietary supplements, we have to look at each one individually and spend a great deal of time determining, is there a quality product out there that we can stock? And if we can find one, we will stock it, but oftentimes, we can't find one, and therefore, we refuse to stock and use it in our patients.
REHMSo, in your opinion, as someone who's been in this field for quite a while, should these supplements be regulated exactly the way pharmaceuticals are?
ERUSHIn my opinion, yes. Because families administer these to their children or take them themselves with the idea that they are treating something. Even though they shouldn't be marketed that way and are not potentially marketed that way, that's obviously the intent of why people are taking them, is they want to get some therapeutic benefit from them. So, they're kind of going on a wing and a prayer. And if they're taking them with the idea that they're going to get a therapeutic effect, we should be able to guarantee that is or isn't true. They should be regulated.
REHMAll right. Dr. Cohen, do you want to jump in?
COHENYes, because on this one, I have a different opinion. I do not think that supplements, or certainly all supplements need to be regulated the same way as drugs. I think that would be excessive. And I think that would leave consumers without access to things that they have had access to for, you know, many, many years. So, I think that what we need is to separate some of these ingredients out. If we're talking about vitamins and minerals, that could be regulated not so different than what we're doing currently. If we have certain limits to the amount of the vitamin.
COHENIf vitamins have warnings on them. So, a high dose of vitamin E says, this can increase prostate cancer. This is information that people can take into account when they're purchasing high dose vitamin E. And consumers can make those decisions for themselves. But when it comes to all the botanicals, and certainly new botanicals, synthetic botanicals, as soon as you start playing with the molecular structure of an ingredient, then it should be regulated just like a new drug.
REHMAll right. Dan Fabricant.
FABRICANTWell, I'm a little concerned with some of the things Sarah said, because I think she's dealing with sick people. As we've stated, this isn't for sick people. These are dietary supplements to supplement the diet, not to treat, cure, mitigate disease as the law clearly stated for. And so applying a sick population, where, I agree, anything that happens to sick kids is tragic, but applying that to the population in general and then saying there's not studies about efficacy.
FABRICANTWell, there's studies about efficacy with calcium, with folic acid, with fish oil, with things like that. A lot of studies. Vitamin D. It seems a little bit of a disconnect. Also, from a public health perspective, the notion that someone in a clinical environment, sure. You've got pretty tight controls over them. But the notion that when people aren't in that environment, they're not gonna do this. As you started the segment with, almost 70 percent of the population takes these products and not to engage them on that and understand it, I think, makes some real challenges.
REHMAnd you come back to the question, how many of these products have been proven to be efficacious? I mean, it's not just what they claim, but it's what I believe they are doing. Julie.
ROVNERAnd it's not just whether or not they're efficacious. I hate to use this as an example, but my horse takes joint supplements, and there's some question about whether there's any oral uptake from the joint supplements, whether they actually get to the joints. So, that was -- this has been a long standing issue. My vet has always shrugged about everybody who feeds their horses, you know, oral joint supplements. And it's like probably not gonna hurt, may or may not help.
REHMAll right. I'm going to open the phones. Let's go to Maria, who's in Harrisburg, Pennsylvania. Hi there.
MARIAHi there. I have two sons in their 20s. And they work out, and they both take Whey Protein. Buy a huge container of it, and I am extremely concerned, because all of their friends, all of them, are doing this. And I'm concerned what's -- if it really is just whey and I think it's probably coming from China. But they get it in big containers, and I am concerned about liver, kidney damage, and should I be concerned?
REHMDr. Cohen.
COHENMaria, that's a great, terrific question. Thanks for asking it. The -- when you're thinking about workout supplements, and they are so, so common among adolescents and young adults, that it's so important for all of us to understand. There's -- I think about it, how I counsel my patients, is that there's three big categories. There's the pre-workout supplements that sold to juice you up before a big workout. Then there's the protein powders and amino acids that are supposed to sort of build your muscles up. And the third category is testosterone like hormones to make you stronger.
COHENSo, at first, I want to strongly discourage the use of anything in the pre-workout supplement category and in the last, testosterone building hormonal category. Now we're left with protein powders and amino acids. These are certainly, of those three categories, the safest. And if someone was insisting, a patient of mine insisting that they wanted to take something, that's what I'd recommend they take.
REHMOkay, but is that all relative? I mean, you're saying it's the safest among the three, but is it actually safe?
COHENWell, whey, we actually export. It's a net positive export, so the whey is probably from the US. It's one of our big exports, so whey protein's the same as what you see in like Carnation Instant Breakfast. So, you know, look at the claims. If the claims are too good to be true, they probably are. Make sure they've got an appropriate address and label to where if there are any incidences, you can contact the firm. The firm is required by law to pass that information along to the agency. So...
REHMNot gonna do you much good if you've already lost one kidney. Go ahead, Julie.
ROVNERInterestingly, the Department of Defense has a good website about diet supplements. Again, because so many members of the military are working out and are taking these supplements to sort of help consumers. And it's available to civilians. And it just walks through. And I should point out also the NIH Office of Dietary Supplements. There are a lot of resources for making, sort of, wiser decisions about what kinds of supplements are useful to take.
REHMAnd you're listening to "The Diane Rehm Show." Sarah, do you want to weigh in on that?
ERUSHI think that I'd like to go back and talk about the vitamins and minerals versus the botanicals. I think what's interesting is we found in investigating the quality of these products is that vitamins and minerals are often classified as foods. And you can't even look at them in terms of dietary supplements, because that information isn't out there. The botanicals are definitely a problem. The botanicals are the ones that we usually stumble up against that patients are bringing in and their patients are taking multiple ones of, that we can't find any information on.
ERUSHAnd back to what everybody said before, in terms of the contamination and the quality, these are all the concerns that we have, and we have found patients taking potentially dangerous products.
REHMAll right. Let's go to Leesburg, Virginia. Hi Noel. You're on the air.
NOELHi Diane. Thank you for taking my call.
REHMSure.
NOELI am a certified personal trainer, and I've been doing this for almost 10 years. And at no point in my profession did I ever advise directly, any of my clients to take any supplements, or protein, whey protein whatsoever. I think we all know that we are living -- we have become a society that's been encouraged to always seek for shortcuts to fix problems. And that is wrong. I was so excited when people were lambasting Dr. Oz for what, you know, he has started doing on his show.
REHMAll right. Thanks for your call. There are lots of ads claiming various things from these vitamins, dietary supplements, powders, whatever. And people believe it, Julie.
ROVNERAbsolutely. And they are -- you know, the Federal Trade Commission, which was sort of the subject of the hearing with Dr. Oz. It was actually not the FDA. Talked about many of the prosecutions that they've made and the, you know, the people that they've gone after. But again, it's this sort of, you know, chase after the fact. Obviously, in the FTC's case, that's basically all they can do. They've been very busy, but you know, you can't open a browser on the internet without seeing multiple ads for, you know...
REHMAnd claims.
ROVNERYes. And claims for, you know, magic weight loss.
FABRICANTOne of the problems at the hearing, that FTC made clear, is they wait until a firm gets big enough to go after. That's, that's a problem. I mean, you've got to have regulators that are there to try and nip it in the bud. There's a wherewithal there. There's a zeal that has to be there. You know, you can sit around and design the perfect car, but if you don't put gas in it, and you don't have -- you know, you're not going to step on the pedal, it's not going to go very far.
REHMDr. Cohen.
COHENThe original thinking with the law was that the claims would be balanced with a disclaimer that Julie has mentioned before, excuse me, which basically says the FDA has not approved this product. So, but there has been some excellent research done to figure out do consumers understand that disclaimer? And does that change the effect of how they think about the claim? Fascinatingly, the results. The results are that the disclaimer has no, zero effect on consumers' belief on the claim. Number one. But, in terms of safety.
COHENBut in terms of efficacy, this is fascinating, if the disclaimer is there saying the FDA -- oh, I'm sorry, not the disclaimer, but if the company puts on a warning saying that this is very potent, be careful, do not take too much. The consumer increases their belief in the efficacy of the product. So, this is often used as a marketing ploy in the workout supplements, putting these dangerous effects on there just to try to sell more of the product. So, it's fascinating.
REHMAll right. We're gonna have to leave it at that. Dr. Pieter Cohen is at the Harvard Medical School. Sarah Erush is at Children's Hospital of Philadelphia. Julie Rovner's Senior Correspondent at Kaiser Health News. Daniel Fabricant, CEO of the Natural Products Association. I want to thank you all so much and caution our listeners. Read those labels carefully and think twice before you start taking lots of supplements. Thanks for listening, all. I'm Diane Rehm.
After 52 years at WAMU, Diane Rehm says goodbye.
Diane takes the mic one last time at WAMU. She talks to Susan Page of USA Today about Trump’s first hundred days – and what they say about the next hundred.
Maryland Congressman Jamie Raskin was first elected to the House in 2016, just as Donald Trump ascended to the presidency for the first time. Since then, few Democrats have worked as…
Can the courts act as a check on the Trump administration’s power? CNN chief Supreme Court analyst Joan Biskupic on how the clash over deportations is testing the judiciary.